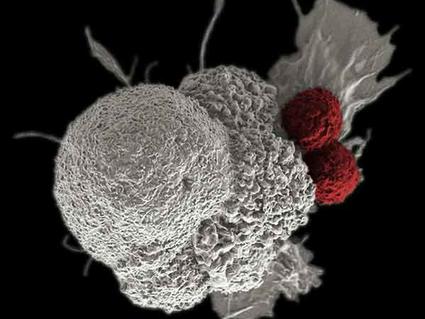
Libtayo™ (cemiplimab) was only approved as an immunotherapy for lung cancer in March 2021. It had previously been marketed in the skin cancer setting, used for squamous cell and basal cell skin cancer. Of course the vast majority of skin cancers do not require systemic treatment with chemotherapy or immunotherapy. Unfortunately some patients do develop progressive disease, or may have skin cancers in difficult locations to operate, such as around the eye. Also some patients do not respond to topical treatment or radiation commonly used in recurrent skin cancer. Here in Southern California we have warm sunshine most of the year, but that also leads to excess skin cancer cases, and occasionally these can become problematic to treat.
Many times dermatologists are not aware of treatment options such as systemic intravenous immunotherapy.
Both Libtayo™ and Keytruda™ are approved in the setting of recurrent or inoperable squamous cell skin cancer. Only libtayo™ is approved in basal cell cancer in the recurrent or unresectable setting.
There are several immunotherapy checkpoint inhibitors for lung cancer. Libtayo™ is approved as a single agent for first line (“up front”) treatment for non-small cell lung cancer (NSCLC) that is metastatic or unresectable and that does not carry a “driver mutation” like ALK, EGFR, or ROS1. Doctors also must check for PD-L1 expression of greater than 50% before offering treatment with libtayo™.
Keytruda™ has a firm advantage in the marketplace since it’s been approved for lung cancer since April 2019. It’s also approved as a single agent for high-expressors of PD-L1 but also approved as a combination with chemotherapy for all non-squamous cell NSCLC, and also approved in many other types of cancer such as renal cell, melanoma, colorectal cancer, and several others.
Another immunotherapy combination, Opdivo™ and Yervoy™ are also approved in the setting of lung cancer, and this duo also makes an appearance in melanoma as well as renal cell carcinoma, liver cancer, and several other tumor types. Opdivo™ is another checkpoint immunotherapy, but Yervoy™ is a CTLA-4 inhibitor
This is a crowded marketplace and perhaps libtayo™’s unique skin cancer story will help differentiate it from a crowded field of other similar treatments.
All of the checkpoint inhibitors carry warnings of adverse effects in the 10% range, so 90% of patients may not have major side effects. This is probably less chance of toxicity compared to conventional chemotherapy and its usual side effects of hair loss, nausea, and weakness. The checkpoint inhibitor effects are thought of as the “itis” type of problems, such as hepatitis (liver inflammation), pneumonitis (lung inflammation), or dermatitis (rashes), among other possible organ systems. Thankfully, these effects are fairly rare. Doctors need to watch patients closely while on these medications and periodically check lab testing.